Patent Foramen Ovale (PFO) Closure Devices Market Expansion Projected To Gain An Uptick During 2027
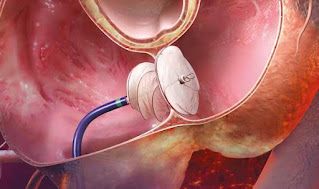
Patent Foramen Ovale (PFO) is a medical condition wherein tissue flap in the wall between left and right atrium remains persistently open, allowing a small amount of blood to pass from the right side of the heart to the left side of the heart. PFO closure device are implantable device used to treat PFO through a minimally invasive, catheter-based technique, implicated in a number of pathological states, including cryptogenic strokes, transient ischemic attacks, migraine with auras, decompression sickness, and severe refractory hypoxemia. PFO closure device market is driven by high incidence rate for ischemic stroke and new product launches.
Patent foramen ovale (PFO) closure device market is majorly driven by the high incidence of ischemic stock. Stroke is the second most common cause of death in the world. According to the American Stroke Association, 2016, annually more than 795,000 people in U.S. suffer from stroke, of which about 87% of stroke are ischemic strokes. Furthermore, increasing number of congenital heart disease is expected to propel growth of the patent foramen ovale (PFO) closure device market. For instance, according to American Heart Association, around 40,000 infants are born with a congenital heart defect in the U.S. annually and around 2 to 3 million individuals are suffering from congenital heart defect. Moreover, increasing adoption of life style related risk factors such as alcohol, smoking, drugs or sedentary life style is expected to elevate the risk of stroke incidence.
Moreover, new product launches are expected to boost growth of the Patent foramen ovale (PFO) closure device market during the forecast period. For instance, in April 2018, W. L. Gore & Associates, Inc. received the US FDA approval for its GORE CARDIOFORM Septal Occluder for PFO closure to prevent recurrent ischemic stroke. The study claimed 77% reduction in recurrent ischemic stroke when PFO closure was combined with antiplatelet therapy, as compared to antiplatelet therapy. Moreover, in 2016, Amplatzer PFO Occluder device was reintroduced after product recall with FDA approval to reduce stroke risk for patients with prior cryptogenic stroke. Furthermore, increasing clinical trials for new device is expected fuel growth of the Patent foramen ovale (PFO) closure device market. For instance, as on 2016, Lifetech Scientific (Shenzhen) Co., Ltd. was conducting its Post Market Clinical Follow-up study for its device named IrisFIT PFO Occluder for the indication of PFO.



Comments
Post a Comment